Are you FMD-ready yet?
The deadline for complying with the EU’s new Falsified Medicines Directive (FMD) is only a few weeks away. From 9th February 2019, pharmacists will be required to scan medicines to confirm their legitimacy before they dispense them to customers.
So if you distribute medicines in the UK or throughout Europe, then you will need to comply with the directive in a number of ways.
What does the FMD mandate?
The directive sets out a number of safety features. Medicines distributors are required to include on their packaging:
- An anti-tampering device, or tamper-evident features
- Unique identifiers – comprising a 2D DataMatrix barcode, plus additional human-readable information
- The EU-wide logo that identifies legal online pharmacies
Additionally, there are stricter rules regarding the importation of pharmaceutical ingredients and wholesale distributors also have to adhere to some more exacting record-keeping requirements.
Are you FMD compliant yet?
Not all businesses will have their solutions completely implemented as yet. But in advance of the 9th February deadline, companies are already being required to notify the Medicines and Healthcare products Regulatory Agency (MHRA) of their intended solution, so that it can be vetted.
If you haven’t done this as yet, then you need to act quickly to establish what you still need to do, and how you will achieve it in advance of the deadline.
FMD and your Warehouse Management System (WMS)
When it comes to ensuring your WMS is compatible with the FMD, there are some key considerations.
Firstly, it needs to be capable of parsing the barcode information that is required on the packaging. To be more specific, these are GS1 2D DataMatrix barcodes, which should include batch number, expiry date and a unique serial number.
Then, a risk-based verification against the UK National Medicines Verification Organisation (SecureMed) must be made at key touch points. For example, checks are made when stock is received, then when it is moved and finally when it is picked for dispatch. This is a two-way communication process. The UK NMVS logs the information and also provides a status report back to you, the medicines distributor. Primary wholesalers that buy from the original manufacturer or Marketing Authorisation Holder (MAH) will not need to undertake risk-based verification as the product is coming from a trusted source.
This allows you to identify the status of the medicine pack. If it’s reported as active, then all is good. If it’s reported as inactive, then you will also be advised of the reason why. It could be that the medicine has been stolen, recalled or withdrawn. Or it may be identified as having already been dispensed, or locked temporarily during an investigation.
Prescription medicines have an additional identifier. For this, your WMS will also need the ability to handle the decommissioning of the medicine. Article 23 of the FMD states that wholesalers may be required to verify the medicine’s safety features and decommission the medicine before supplying it to organisations that are not dispensing hospitals or pharmacies. For example, a wholesaler may need to decommission medicines that it supplies to universities, research bodies, vets and government bodies.
The Balloon One solution for meeting FMD requirements
Balloon One has a solution for wholesalers that meets the FMD requirements. Via our Springboard Server, we connect your ERP system and your WMS to the UK NMVS.
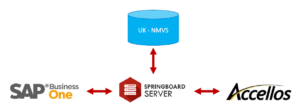
No manual administration is required as the system is fully integrated.
The risk based verifications are all carried out automatically, again without any manual processes or intervention. There is full GTIN, barcode parsing and transactional history recording entirely out of the box. And a single database manages serialised warehouse safeguards such as track and trace, allocation and automatic management.
The Balloon One solution has a minimal impact on operations and can be quickly implemented.
Are you on track to comply with the FMD?
If you haven’t yet done everything you should to comply with the FMD, there is still time. Just.
And if you’re panicking and haven’t finalised your solution yet, give us a call on 020 8819 9071 or get in touch.
